SGPGI Breast Cancer protocols have been prepared with the following in mind:
- Views of Global experts
- Perception of the local experts and patients
- Socio-economic, health care logistics in India
- WHO/MoH guidelines
Department of Endocrine & Breast Surgery Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow, India
Evidence-Based Pragmatic SGPGI Breast Cancer Management Protocols (Summary)
Background
Breast cancer management in country like ours with resource limitation and uneven income distribution has to be approached differently from the industrialized world. The stage at disease presentation and pathology are different, so are the socio-economic compulsions of the patients, necessitating emphasis on efficacious, yet safe and cheap management strategies. A pragmatic approach to individual breast cancer patient based on sound scientific evidence, yet keeping the socio-economic realities and infrastructural and manpower compulsions of SGPGI have been worked out over period of many years. Guidelines foprom various professional bodies, meta-analysis, systematic reviews and RCT’s, along with interpretations of contemporary data from faculty and residents of this department as also of collaborating departments of Radiation Oncology, Pathology, Nuclear Medicine and Radio-diagnosis have formed the basis of these guidelines to a large extent. The first formal SGPGI Breast cancer protocols were formulated in late 2001. Since that time, two major revisions have been made. A summary of the third revised version of SGPGI Breast Cancer protocols is provided here.
Clinical presentation of breast carcinoma at SGPGIMS Lucknow include
Breast Lump
- Usually painless progressive
- Occasional nipple discharge
- Ulcerated growth
Metastatic symptoms like weight loss, bone pain, jaundice or hemoptysis
Operated elsewhere (various degrees of surgical intervention)
Screen detected (rare)
Patients presenting for hospital based screening, out of concern for cancer usually have-
- Breast pain
- Breast nodularity
- Women with family history
- Patients referred for screening/evaluation before or during HRT
Approach to breast lump/suspected breast malignancy
A detailed history including
- Number of off-springs and adequacy of breast feeding
- Menopausal status, history
- Onset, duration and progress of lump
- Associated nipple discharge
- History of trauma to breast, fever
- Use of HRT, OCP
- Family history of breast carcinoma, ovarian malignancy and other related tumors in first and second degree relatives
Diagnostic investigation of a suspected malignancy
Triple test
- Clinical breast examination
- Fine needle aspiration cytology
- Mammography/USG breasts
For patients having prior intervention elsewhere, review of the histology/cytology slides & Blocks.
Based on the above initial workup, a cytologically proven or suspected breast cancer is staged clinically according to the TNM- AJCC 2002* staging system of breast carcinoma
(* Refer to 6th edition of AJCC manual of TNM staging, also available in this course manual in later article)
Clinical stage grouping is done for ease of communication and management planning, as follows:-
Early Breast Cancer
Small operable tumors (<5 cm), nodal status N0/N1, M0 Breast conservation possible
Large Operable Cancers
Large operable tumor (>5 cm), nodal status is N0/N1, M0 Prognosis is similar to stage II disease Mastectomy is possible, breast conservation is difficult
Locally advanced breast carcinoma
Mostly stage III disease: T4, N2/ N3, M0 Considered inoperable, will require neo-adjuvant systemic treatment
Metastatic disease
Evidence of metastasis (other than regional lymph nodal metastases) Treated with primary systemic treatment/palliative measures alone
Investigative work-up after clinical staging:
Following minimal metastatic workup after a working diagnosis and staging is done. In selected patients, other symptoms/signs directed test may be employed-
X ray Chest- PA view
Blood chemistry including serum Alkaline phosphatase, LFT
Mammography if not done earlier.
If >T2 or >N1 disease, symptomatic, raised serum alkaline phosphatase- also include
-99mTc MDP Skeletal Scan
-USG abdomen- to look for metastatic deposits
Clinical staging is upgraded with any added information from imaging.
Treatment protocol for early breast cancer
Early Breast Cancer
T1/T2, N0/N1, M0 disease
Stage I, IIA, IIB (T2N1)
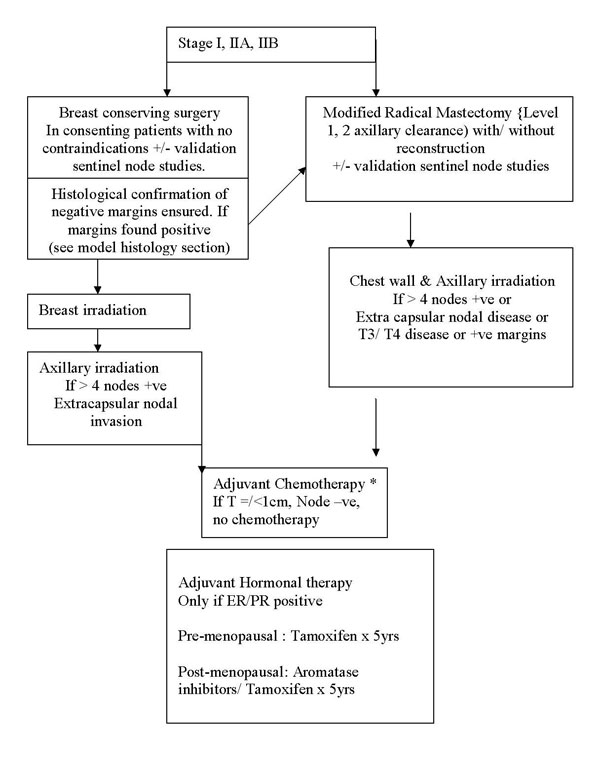
Treatment protocol for locally advanced breast cancer
Locally advanced (and Large operable) breast cancer
Stage IIIA, IIIB, IIIC, and IIB (T3N0M0)
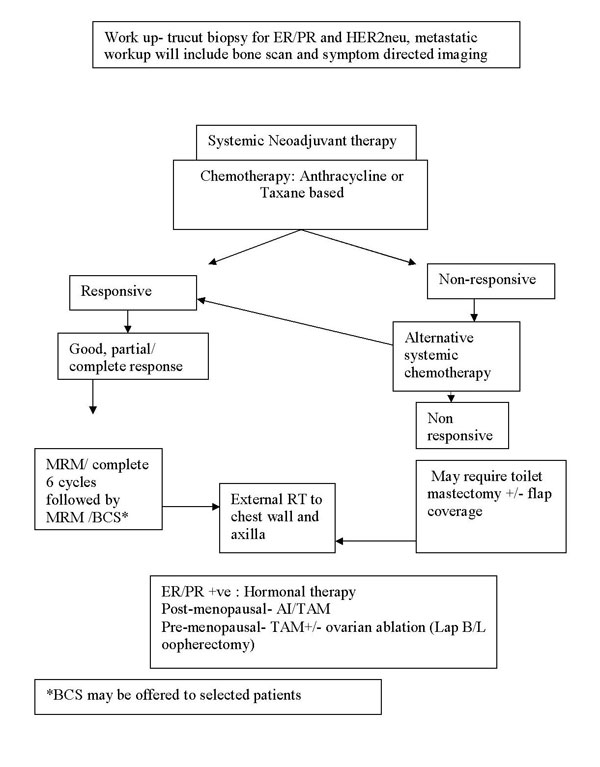
Treatment protocol for Metastatic Ca Breast:
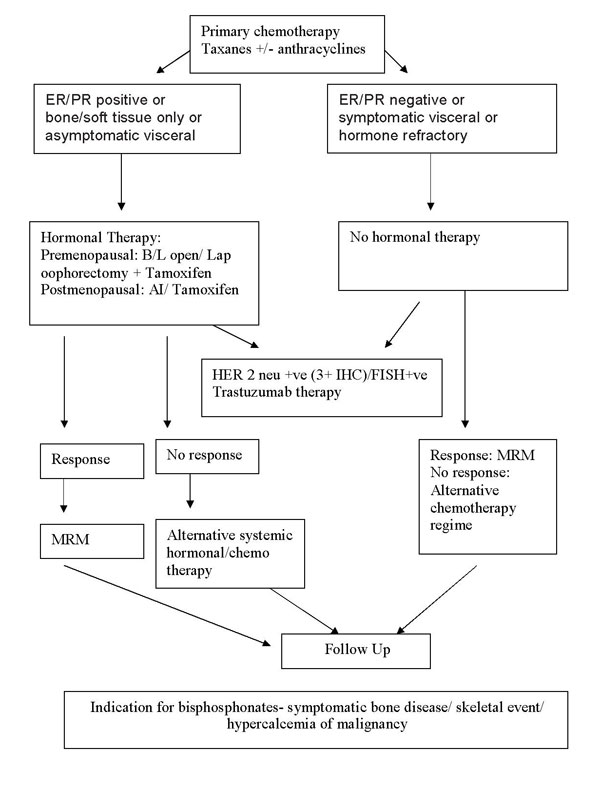
Chemotherapeutic regimen and agents used commonly
| Sr. No. | Group | Drug | Dose |
|---|---|---|---|
| Antiestrogen | Tamoxifen | 20mg PO OD | |
| Aromatase inhibitors | Letrozole Exemestane |
2.5mg PO OD | |
| HER 2 monoclonal antibody | Trastuzumab | 4mg/kg loading dose 2mg/Kg/week maintenance till disease progression/1yr/critical toxicity appears |
Hormonal agents/targeted therapy used
| Regimen | Cycle Interval | Drugs | Dose |
|---|---|---|---|
| CAF | q 21 d | Cyclophosphamide | 600mg/m2 IV Day 1 |
| Doxorubicin | 60mg/m2 IV Day 1 | ||
| 5 Flurouracil | 600 mg/m2 IV Day 1 | ||
| CEF | q 21 d | Cyclophosphamide | 500mg/m2 IV Day 1 |
| Epirubicin | 100mg/m2 IV Day 1 | ||
| 5 Flurouracil | 500mg/m2 IV Day 1 | ||
| AT | q 21 d | Adriamycin | 60mg/m2 IV Day 1 |
| Docetaxel | 100mg/m2 IV Day 1 | ||
| TAC/ TEC | q 21 d | Docetaxel | 100mg/m2 IV Day 1 |
| Doxorubicin/ Epirubicin | 50mg/m2 IV Day 1 | ||
| Cyclophosphamide | 500mg/m2 IV Day 1 |
Follow up Protocol
First visit after completing the treatment (Surgery, chemo, and radiotherapy): starts 3 months after completion of treatment or 1 yr after initial evaluation which ever is earlier.
- Clinical breast examination
- Hemogram
- Blood chemistry incl s-ALP, LFT, Ca
- CA 15-3 (selective)
- X ray chest
- ECG/ECHO to r/o CT/RT toxicity
- Bone mineral densitometry
6 months post treatment
- Clinical breast examination
- Alk PO4
- X ray chest PA
- Symptom/chemistry directed tests
1 year after completing initial treatment
- Clinical breast examination
- Blood chemistry incl S Calcium, Alk PO4
- X ray chest PA
- Mammogram
- USG abdomen
- Tc 99 MDP Bone Scan
Model histology report includes
| Patient name | : |
| Age/sex | : |
| Central registration number | : |
| Side - Left/Right | : |
| Date of reporting | : |
Type of specimen
Breast specimen - Wide local excision/Segmental excision/Mastectomy Axillary Specimen- Axillary clearance/Axillary sampling/Sentinel node(s)
Gross Histology
No of lesions/Size of lesion/Site of lesion
No of nodes dissected/grossly significant nodes/Sentinel nodes (no of blue/hot/both blue and hot)
Microscopy
Tumor histology/grade of tumor/vascular or lymphatic invasion/margin status of specimen No of nodes positive/extra-lymphatic spread/sentinel node status
Immunohistochemistry
Hormonal receptor (ER/PR) and HER2neu status